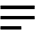Mechanisms on accelerating hydration of alite mixed with inorganic salts in seawater and characteristics of hydration products
Synopsis
The main inorganic salts in seawater increase the saturated Ca concentrations in solutions, contributing to the hydration of alite. This research will help the usage of seawater in the construction of a sustainable material.
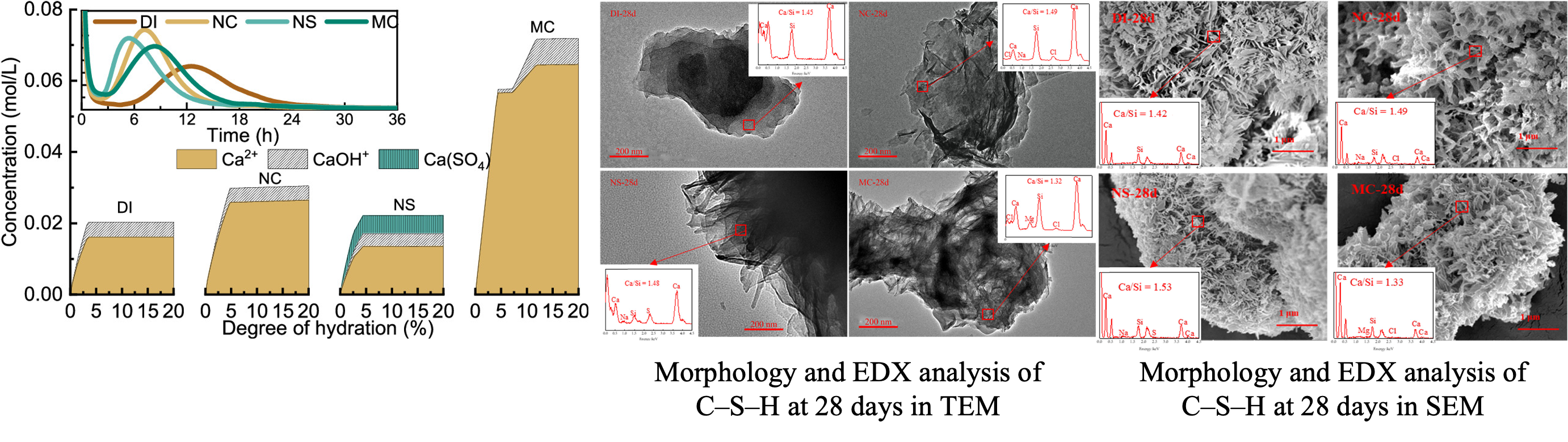
Interest in exploring the use of seawater as the mixing water for preparing concrete is increasing due to the lack of freshwater in some coastal regions and remote islands, where seawater is more accessible. However, up to now, the mechanism of the accelerating effect of seawater on the hydration of portland cement (PC) remains unclear. In this study, alite, a major clinker phase in PC, was hydrated with common salt solutions (NaCl, Na2SO4, and MgCl2) in seawater to explore the mechanism of acceleration. The heat release peaks of the salt-added systems shifted to an earlier hydration time with a higher peak value, which indicated the faster hydration rate of alite pastes compared to the deionized (DI) water system. The addition of the single salts was found to increase the concentration of Ca species in solutions, contributing to the increased formation of calcium–silicate–hydrates (C–S–H) and portlandite at early ages. In the Na2SO4 system, gypsum was the new hydration product, while brucite was formed in MgCl2 systems, which caused the sharp decrease of Mg species in the solution. The morphology of the early formed C–S–H was changed with the addition of the salts, and the C–S–H were characterized as thinner and longer fibers. At later ages, the incorporation of the single salts lowered the polymerization degree of C–S–H, but no noticeable morphological change was observed.