Innovative Spring-structured Elastic Surface for Spontaneous Ejection of Freezing Droplets
Other Articles

Bio-inspired elastic-to-kinetic energy transformation facilitates innovative scalable surface design in preventing ice adhesion.
Study conducted by Prof. Zuankai WANG, Prof. Haimin YAO
and their research team
Preventing the accumulation of freezing droplets on surfaces is a significant challenge in many practical applications, including energy harvesting, thermal management, self-cleaning, and anti-icing coatings. In transportation, this issue creates hazardous conditions on infrastructures such as roads and railways. Additionally, the accumulation of freezing droplets endangers the manoeuvrability and safety of aircraft and vehicles. In the electricity sector, ice accumulation on power lines and wind turbines can cause outages and decreased efficiency. In manufacturing, ice formation can obstruct equipment, resulting in malfunctions. Furthermore, communication systems experience signal disruptions due to ice on antennas. Traditional de-icing methods often require energy consumption, or involve chemicals that harm the environment.
Innovative material science and surface engineering have introduced methods to delay ice nucleation and propagation, as well as ice adhesion. One approach involves developing anti-icing coatings that create hydrophobic surfaces, preventing water droplets from adhering and freezing. Another method utilises phase-change materials (PCMs) that absorb heat during freezing, delaying ice formation. However, the hydrophobic surfaces usually function well only under specific conditions (e.g. under vacuum conditions), while developing high performance PCMs can be costly, which limits their application.
Inspired by an elastic-to-kinetic energy transformation of the fungus Pilobolus kleinii, which disperses spores spontaneously (Figure 1), a research team led by Prof. Zuankai WANG, Associate Vice President (Research and Innovation), Kuok Group Professor in Nature-Inspired Engineering, Chair Professor of Nature-Inspired Engineering of Department of Mechanical Engineering, and Prof. Haimin YAO, Associate Professor and Associate Head (Teaching and Learning) from the same department, harnessed the mechanical properties of freezing water droplets and proposed a structured elastic surface with spring-like pillars and wetting contrast that enables the spontaneous ejection of freezing droplets [1]. The freezing of water naturally leads to approximately 9% volume expansion under normal atmospheric conditions, providing an alternative energy source for droplet ejection. To accelerate energy transformation to drive the ejection of freezing droplets, the research team designed a structured elastic surface (SES) that exploits the volume expansion effect of water when it freezes.
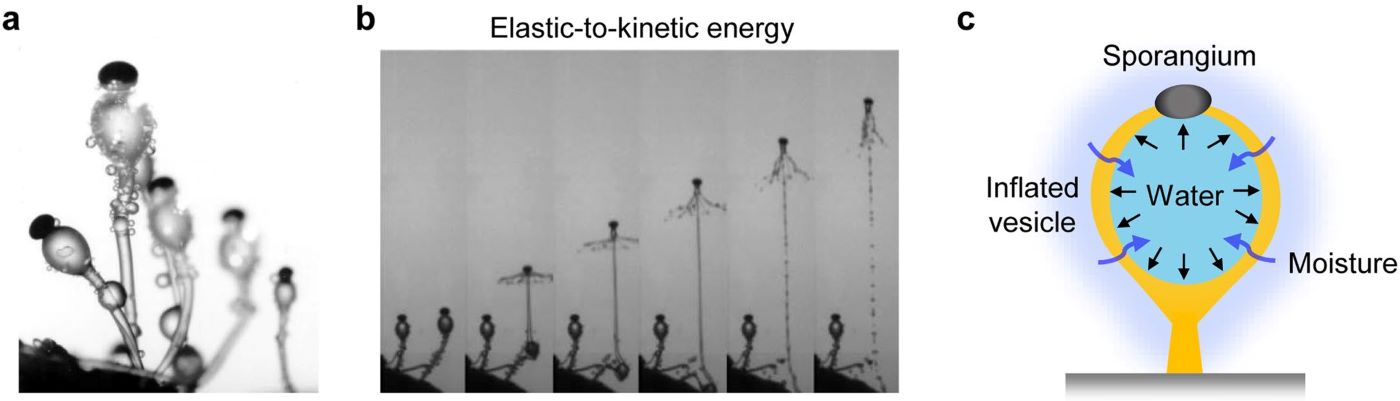
Figure 1. Energy conversion for spore dispersal in the fungus (Pilobolus kleinii).
a, Photograph of the fungus (Pilobolus kleinii). b, Snapshots showing the spore dispersal behavior of the fungus driven by the conversion of elastic-to-kinetic energy. c, Schematics of the mechanism of the energy conversion process in the fungus. In brief, the vesicle of the fungus stores elastic energy by absorbing ambient moisture. Once a critical internal pressure is reached, the vesicle explodes, rapidly releasing the stored elastic energy and ballistically discharging the spores from its tip.
In their study published in Nature Chemical Engineering as a front cover, the proposed SES is made of elastic polydimethylsiloxane using a simple cast-moulding process. This comprises three key components: a smooth substrate with a radius of 0.5mm, a central deformable spring-like pillar with a radius of 0.09mm and a height of 0.4mm, and a micro-patterned superhydrophobic base surrounding the substrate (Figure 2). The smooth substrate serves as the initial attachment point for droplets, while the spring-like pillar acts as the core component for energy storage and release. The micro-patterned base facilitates the spontaneous movement of droplets onto the smooth substrate, where they are captured by the spring pillar.
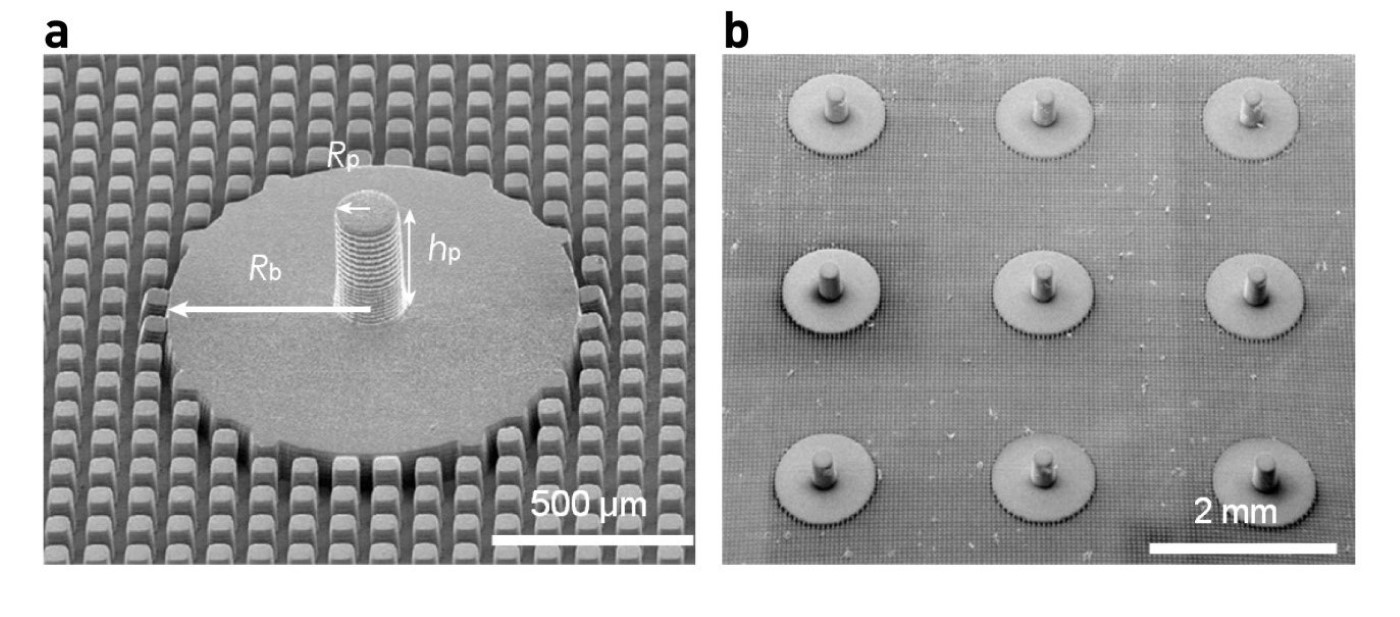
Figure 2. Schematic design of the structured elastic surface.
a, Scanning electron microscopy image showing the structures of the SES, consisting of a pillar with radius Rp = 0.09mm and height hp = 0.4mm, a smooth base with radius Rb = 0.5mm and a micropatterned substrate surrounding the base. b, Scanning electron microscopy image of the SES arrays.
The ejection behaviour of a freezing droplet on SES results from a two-stage energy conversion process: energy storage (Stage I) and energy release (Stage II). When a droplet lands on the SES and begins to freeze, the water inside the droplet expands, compressing the spring-like elastic column located at the centre of the base. During this process, the volume expansion work of the droplet is converted into elastic potential energy, which is stored in the elastic column. As the droplet continues to freeze, the elastic column becomes increasingly compressed, and the stored elastic potential energy is released within milliseconds, transforming into kinetic energy. This allows the droplet to overcome adhesion to the surface and be ejected spontaneously (Figure 3).
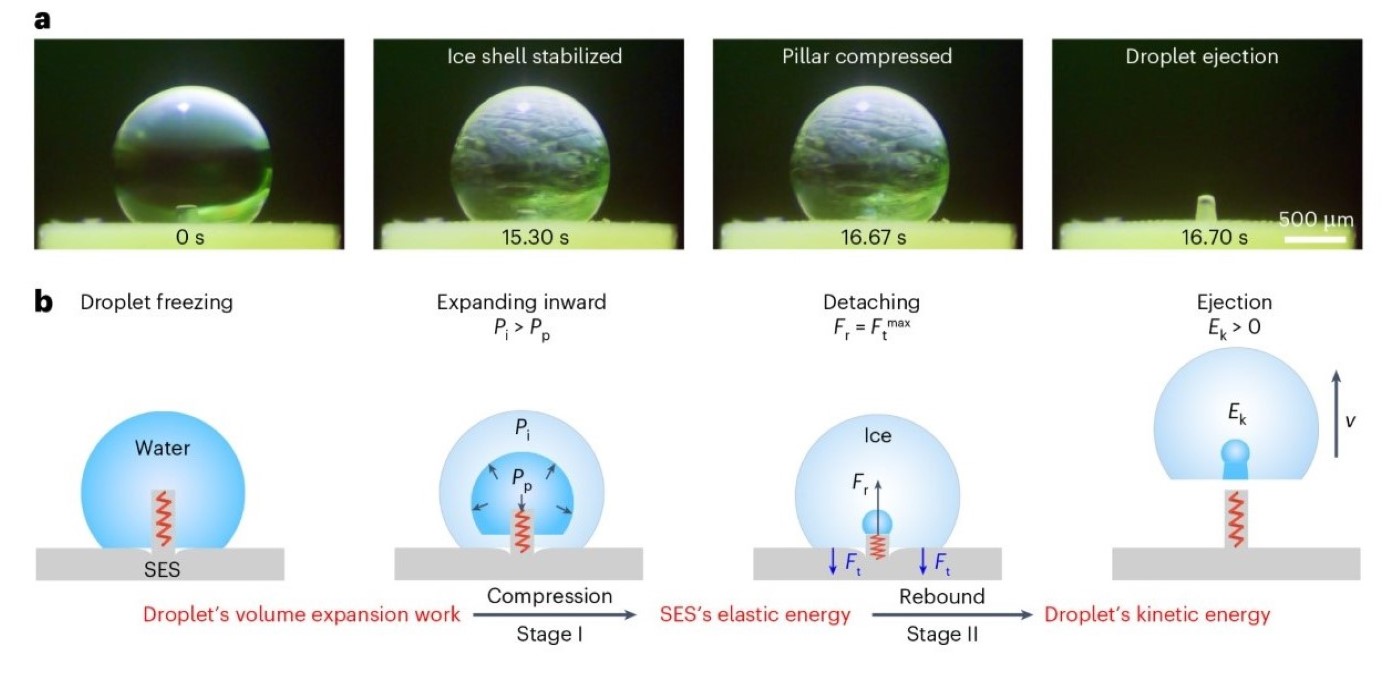
Figure 3. Characterisation and theoretical modelling of the dynamic interactions between the freezing droplet and the SES.
a, Visualisation of a 2 μl dyed freezing droplet ejection process on the SES sample with Rb = 0.5 mm and Kc = 5.9 MPa. b, Schematic of the theoretical model illustrating the two-stage energy conversion process for freezing droplet ejection by considering the pillar as a spring for energy storage and release. The two-stage energy conversion process is shown: Stage I, converting the droplet’s volume expansion work into SES’s elastic energy via pillar compression; Stage II, converting SES’s elastic energy into the droplet’s kinetic energy via pillar rebound. Pi, the pressure-bearing capacity of the ice shell of the freezing droplet; Pp, the internal pressure of the freezing droplet; Fr, the repelling force generated by the compressed pillar; Ft(max) , the maximal traction force; Ek, the kinetic energy gained by the freezing droplet.
To assess the effectiveness of the surface design, the research team conducted an experiment involving multiple icing cycles on SES with 3 × 3 arrays, alongside a smooth control sample made from the same materials. In each cycle, nine droplets were deposited on both surfaces and the mass of residual ice was measured after 30 seconds of freezing. After six consecutive cycles, the SES arrays exhibited significantly less ice accumulation, retaining only about 33% of the residual ice compared to the control. Through further experiments, the research team additionally observed that the ejection behaviour of the droplet could be influenced by several factors, including droplet size, the elastic modulus of the SES, the radius of the base, and the ambient temperature.
This innovative design not only enhances our understanding of multiphase freezing dynamics but also introduces a novel approach without any energy input to reducing ice adhesion. Its potential for scalable manufacturing through a numbering-up strategy opens up applications in de-icing and inspires the development of droplet-based energy generators and soft robotic catapults.
The research is supported by the Research Grants Council of Hong Kong (Grants 15237824, Z.W., SRFS2223-1S01, Z.W., C1006-20W, Z.W., 11218321, Z.W., 11219219, Z.W.), the Tencent Foundation through the XPLORER PRIZE (Z.W.) and the Meituan Foundation through the Green Tech Award (Z.W.). The data source is available at: https://www.nature.com/articles/s44286-024-00150-1#Sec18. Code can be obtained from the corresponding authors upon request.
Prof. Wang was named as a "Highly Cited Researcher" by Clarivate Analytics for three consecutive years from 2022 to 2024. He was ranked among the top 2% most-cited scientists worldwide (for career-long) by Stanford University in the field of nanoscience & nanotechnology for two consecutive years (2023 and 2024). Prof. Wang has been bestowed the 2024 Nukiyama Memorial Award by the Heat Transfer Society of Japan for his significant contributions to thermal science and engineering. Previously, in 2022, Prof. Wang was awarded the inaugural RGC Senior Research Fellowship, as well as the Bank of China (Hong Kong) Limited Science and Technology Innovation Prize for his exceptional accomplishments in the field of "Advanced Manufacturing".
Prof. Yao was ranked among the top 2% most-cited scientists worldwide (for career-long) by Stanford University in the field of mechanical engineering & transports for four consecutive years from 2021 to 2024. In recognising his continuously contributions in engineering research, Prof. Yao was elected as the Fellow of the American Society of Mechanical Engineers in 2024.
| Reference |
|---|
[1] Zhang, H., Zhang, W., Jin, Y., Wu, C., Xu, Z., Yang, S., Gao, S., Liu, F., Xu, W., Wang, S., Yao, H, and Wang Z. (2024). Freezing droplet ejection by spring-like elastic pillars. Nat Chem Eng https://doi.org/10.1038/s44286-024-00150-1
 | Prof. Zuankai WANG |
 | Prof. Haimin YAO and Learning), |




